Detecting “foreign” RNA in the cytoplasm is one of the first defense mechanisms of the cell against virus infection. Specialized sensors called pattern recognition receptors patrol the cytoplasm for RNA molecules that are chemically different from the cell’s own RNA, for example, because they are double-stranded instead of single-stranded. Recognition of double-stranded RNA (dsRNA) leads to a signaling cascade that induces various defense mechanisms against the viral invader. In a previous publication, our lab identified a kinase called TAOK2 as a strong binder of dsRNA and showed that in the absence of TAOK2, cells are less able to mount an immune response to virus infection.
Before our paper, TAOK2 was mostly known for its role in neuronal development, and we don’t really understand what it is doing after binding to viral dsRNA to impact the immune response. Therefore, Lara Rheinemann, a postdoctoral researcher in our lab, has set out to answer the following questions:
How does TAOK2 bind to RNA and what is the result of this?
We were the first to show that TAOK2 binds to RNA and that this binding activates the kinase activity of the enzyme. However, we still don’t really know how it does that, especially since the protein does not contain a conventional RNA-binding domain. In collaboration with Carina de Oliveira Mann’s lab, Lara will investigate what RNA molecules are bound by TAOK2 and what the functional consequences are. Does the binding lead to a conformational change? Do several TAOK2 molecules multimerize on the RNA? State-of-the-art cryo-electron microscopy at Carina’s lab and classical biochemistry methods will help us find out!
How does TAOK2 influence antiviral signaling?
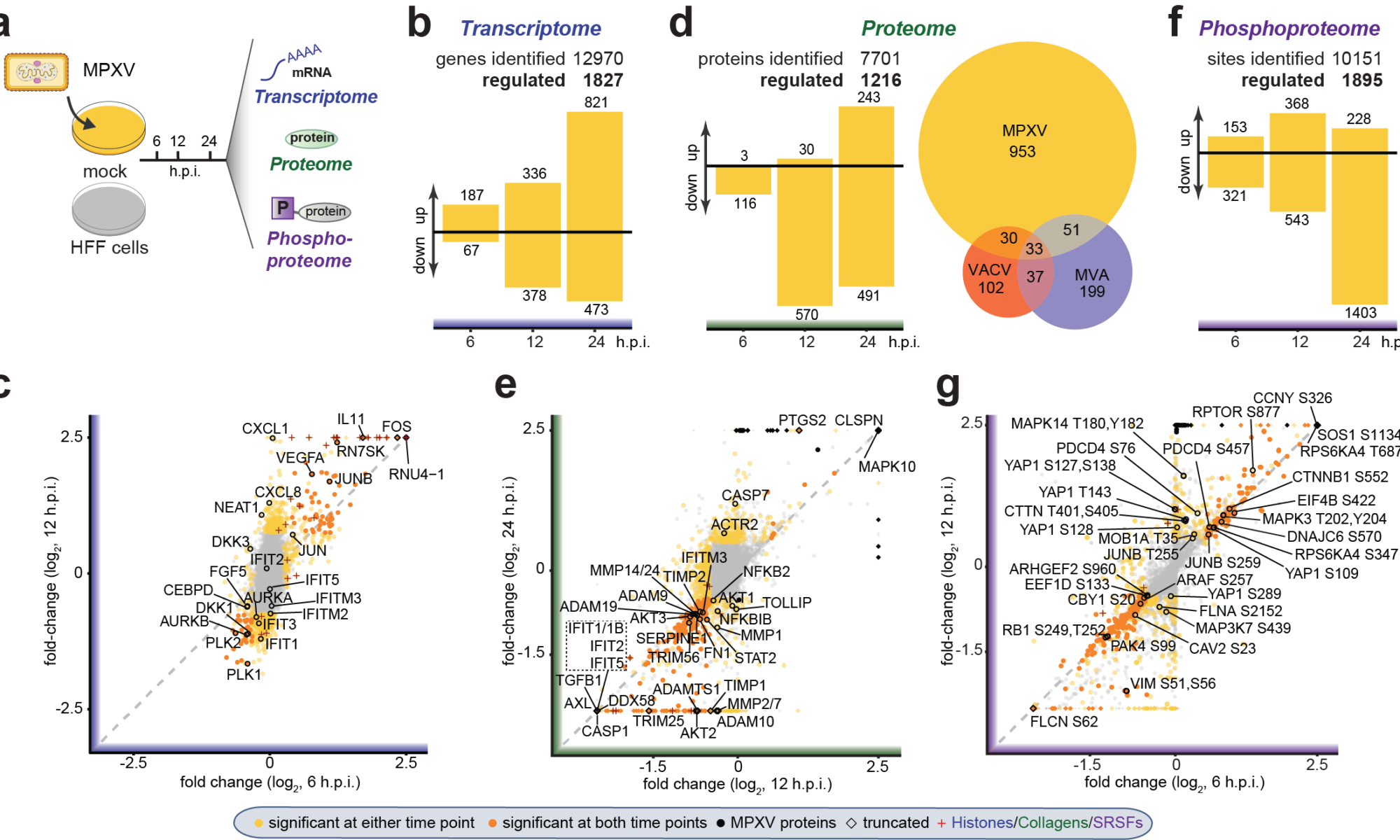
We know that in the absence of TAOK2, the innate immune response against viral infections is dampened substantially. These signaling events involve many different players, from other pattern recognition receptors such as MDA5 and RIG-I to transcription factors that are activated by the resulting signals. Lara will use multilevel systems biology approaches featuring transcriptomics, phosphoproteomics, and proteomics methods to tease apart this complex network and figure out the role of TAOK2 in all of this.
Does this matter in vivo?
To transfer our results from the tissue culture dish into a more physiologically relevant model, Lara will collaborate with Søren Paludan’s lab in Denmark, who will test if mice that lack TAOK2 can still efficiently raise an immune response to virus infection.
The EU was convinced that this project is worthwhile and awarded Lara a Marie Skłodowska-Curie Postdoctoral Fellowship to support her work!
The project will run from September 2023 to August 2025, and you can find it here on CORDIS.

Text by Lara.